How Are Differences in Properties Among the Transition Elements Explained
Many transition element compounds are brightly colored due to the inner-level d electron transitions. They can form several states of oxidation and contain different ions.

Transition Metals Elements Definition List Properties Transition Metal Electron Configuration Ionization Energy
We know that electronic configurations of transition elements is invariably n-1d 110 ns 0 or 1 or 2 which indicates that i the electronic configurations of transition elements differ from one another only in the number of electrons in d orbitals in the n-1th shell and ii the number of electrons in the outermost shell ns is invariably 1 or 2.

. The physical and chemical properties of transition elements differ from the main group elements s-block. Densities and Metallic Radii. Each has specific chemical and physical properties.
In general the next higher s sublevel is already filled or has one electron missing. Most compounds of transition metals are paramagnetic whereas virtually all compounds of the p-block elements are diamagnetic. The ability to become magnetic temporarily in.
Transition metals have typical metallic qualities such malleability ductility high tensile strength and metallic lustre. Inner transition elements are in the f-block and in the f-orbital have valence electrons. Trends in the metallic characteristics of the transition elements on the other hand can be seen.
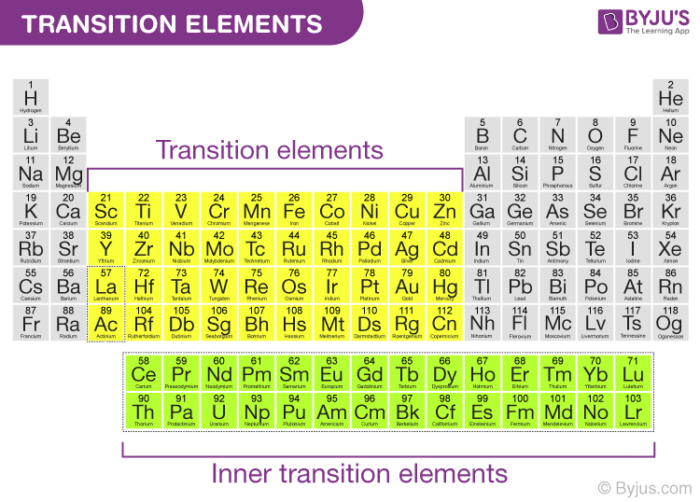
Transition metals are in the d block and the f block of the periodic table. Properties of transition elements are discussed below. The d-block elements are called transition metals.
This happens as each additional electron enters the penultimate 3d shell. The lanthanides and the actinides at the bottom of the table are sometimes known as the inner transition metals because they have atomic numbers that fall between the first and second elements in the last two rows of the. This colour is explained by the d-d transition of electrons.
These properties of the transition elements are listed below. The transition metals are the metallic elements that serve as a bridge or transition between the two sides of the table. The transition elements are found in groups IIIA-IIB new groups 3-12.
All transition elements exhibit similar properties because of the identical electronic configuration of their peripheral shell. They have a high chargeradius ratio. These elements are transition metals.
This creates an effective shield between the nucleus and the outer 4s shell. However the atom sizes of the elements in the third transition series are virtually the same as those of. These elements all of which are metals include some of the best-known names on the periodic table iron gold silver copper mercury zinc nickel chromium and platinum among them.
In a nutshell Stoner criterion states that magnetism emerges as a compromise between kinetic energy and exchange interaction. These metals tend to be very hard. Compared to other metals most transition metals have.
A number of other transition elements are probably somewhat. The difference between representative elements and transition elements is that representative elements are the chemical elements in the group 1 group 2 and in the groups from 13 to 18 whereas transition elements are chemical elements in group 3 to group 12 including. Transition elements are ALL solid metals except.
I The atomic sizes of the elements of the first transition series are smaller than those of the heavier elements of 2nd and 3rd transition series. While magnetism in isolated transition metal ions is governed by Hunds rules the magnetic properties of bulk transition metals are determined by band magnetism and Stoner criterion 18. They have a variety of oxidation states.
The peripheral shell configuration of these elements is ns 2. They usually combine to form coloured compounds. They tend to crystallize and are generally good conductors of heat and electricity.
The transition elements therefore exhibit many oxidation states. According to the IUPAC a transition metal is any element with a partially filled d electron sub-shell. The transition elements are in the d-block and in the d-orbital have valence electrons.
The transition metals as a group have. High density and hardness. High melting point group 1 metals have low melting points hard group 1 metals are soft high density group 1 metals have lower densities Chemical properties.
Thus Sc is a rather active metal whereas Cu is much less reactive. The transition elements are the elements that make up Groups 3 through 12 of the periodic table. The properties of the elements of the first transition series differ from those of the heavier transition elements in many ways.
The electronegativities of the first-row transition metals increase smoothly from Sc χ 14 to Cu χ 19. The density and hardness of transition elements are high due to the small size of their atoms and the strong metallic bonding. Properties of Transition Elements.
Some properties of transition elements are different from those of the metals in group 1. They are frequently paramagnetic. These elements form coloured compounds and ions.
Transition metals look shiny and metallic. On the periodic table main group elements are found in groups 1 2 and 13-18 while transition metals are found in groups 3-12. The transition metals are malleable easily hammered into shape or bent.
The general properties of. The transition elements general properties are as follows. The biggest difference in properties however is transition metals paramagnetic property.
Properties of transition elements at room tempertature. There is a relatively low gap in energy between the possible oxidation states of these elements. The electronegativities of the first-row transition metals increase smoothly from Sc χ 14 to Cu χ 19.
Most transition metals are grayish or white like iron or silver but gold and copper have colors not seen in any other element on the periodic table. They are the Lanthanides and the Actinides. Thus Sc is a rather active metal whereas Cu is much less reactive.
The three main differences are. Most compounds of transition metals are paramagnetic whereas virtually all compounds of the p-block elements are diamagnetic. They are typically metals with a high melting point.
This describes groups 3 through 12 on the periodic table although the f-block elements lanthanides and actinides below the main body of the periodic table are also transition metals. These elements are characterized by having unfilled d sublevels.
Transition Elements General Properties And Trends With Faqs

General Properties Of Transition Elements D Block Videos Examples

Properties Transition Elements 2 Transition Metal Transition Element Transitional

0 Response to "How Are Differences in Properties Among the Transition Elements Explained"
Post a Comment